Abstract
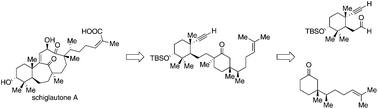
Herein is described a convergent enantioselective route to an advanced intermediate in the synthesis of schiglautone A, a Schisandra triterpenoid with an unusual architecture. The synthetic route to this intermediate displaying 6 of the 7 stereocenters builds upon two fragments, an aldehyde elaborated from the Wieland–Miescher ketone, and a ketone. The preparation of the latter features a lithiation–borylation enzymatic resolution sequence, which led to the formation of the desired product with high enantio- and diastereoselectivities. After aldol coupling of the two fragments, the final quaternary stereocenter was installed by cyclopropane opening. The functionalized intermediate was isolated as a single diastereoisomer and thus offers a valuable starting point for further synthetic exploration.
- This article is part of the themed collection: Celebrating excellence in research: women of organic chemistry


 Please wait while we load your content...
Please wait while we load your content...