One-pot sequential reaction to 2-substituted-phenanthridinones from N-methoxybenzamides†
Abstract
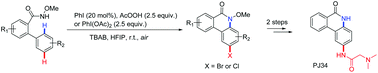
The sequential use of a hypervalent iodine reagent leads to the one-pot synthesis of 2-bromo/chloro-phenanthridinones via an amidation of arenes followed by a regioselective halogenation reaction. These consecutive C–H functionalization reactions can be used efficiently to construct 2-substituted-phenanthridinones at room temperature with good to high yields. Application of the current method is highlighted by the concise synthesis of the natural product PJ34.


 Please wait while we load your content...
Please wait while we load your content...