Structurally diverse arene-fused ten-membered lactams accessed via hydrolytic imidazoline ring expansion†
Abstract
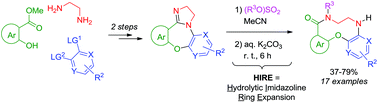
Imidazoline-fused [1,4]oxazepines (prepared in two simple steps from methyl 2-hydroxyaroates, ethylene diamine and bis-electrophilic aromatics) undergo a facile, good-yielding hydrolytic imidazoline ring expansion (HIRE) upon N-alkylation and treatment with aqueous K2CO3. The resulting arene-fused ten-membered lactams significantly add to the contemporary arsenal of small-molecule scaffolds where medium-sized ring systems are severely underrepresented.


 Please wait while we load your content...
Please wait while we load your content...