A highly enantioselective Friedel–Crafts reaction of 3,5-dimethoxylphenol with nitroolefins mediated by a bifunctional quinine derived thiourea catalyst†
Abstract
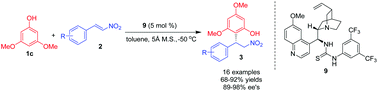
A useful enantioselective Friedel–Crafts reaction of 3,5-dimethoxylphenol with nitroolefins catalyzed by a bifunctional quinine derived thiourea catalyst 9 was developed. The Michael addition products could be obtained in good to high yields (68–92%) and with excellent enantioselectivities (89–98% ee). Such a reaction is exceptionally attractive in virtue of its simple protocol, ready availability of the starting materials, and practical applications of the products.


 Please wait while we load your content...
Please wait while we load your content...