Stereoselective synthesis of novel adamantane derivatives with high potency against rimantadine-resistant influenza A virus strains†
Abstract
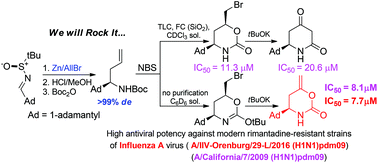
A series of (R)- and (S)-isomers of new adamantane-substituted heterocycles (1,3-oxazinan-2-one, piperidine-2,4-dione, piperidine-2-one and piperidine) with potent activity against rimantadine-resistant strains of influenza A virus were synthesized through the transformation of adamantyl-substituted N-Boc-homoallylamines 8 into piperidine-2,4-diones 11 through the cyclic bromourethanes 9 and key intermediate enol esters 10. Biological assays of the prepared compounds were performed on the rimantadine-resistant S31N mutated strains of influenza A – A/California/7/2009(H1N1)pdm09 and modern pandemic strain A/IIV-Orenburg/29-L/2016(H1N1)pdm09. The most potent compounds were both enantiomers of the enol ester 10 displaying IC50 = 7.7 μM with the 2016 Orenburg strain.


 Please wait while we load your content...
Please wait while we load your content...