Rapid access to N-(indol-2-yl)amides and N-(indol-3-yl)amides as unexplored pharmacophores†
Abstract
Preparation of N-(indol-2-yl)amides and N-(indol-3-yl)amides are scarce in the scientific literature due to unstable intermediates impeding current reported syntheses. We have employed cheap and readily available substrates in the Curtius rearrangement of indole-3-carboxazide to afford N-(indol-3-yl)amides. The reaction is observed for alkyl and aryl carboxylic acids and both N-substituted or 1H-indole derivatives are tolerated. This approach was extended to the preparation of N-(indol-2-yl)amides from the corresponding indole-2-carboxazides.
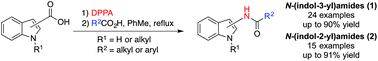


 Please wait while we load your content...
Please wait while we load your content...