Enantiospecific total syntheses of meroterpenoids (−)-F1839-I and (−)-corallidictyals B and D†
Abstract
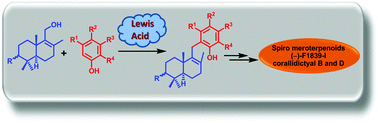
Enantiospecific total syntheses of spiromeroterpenoid natural products (−)-F1839-I and (−)-corallidictyals B and D were achieved using the environmentally benign and highly atom economical Lewis acid catalysed Friedel–Crafts reaction and a highly regio- and stereoselective spirocyclic C–O bond formation reaction.

 Please wait while we load your content...
Please wait while we load your content...