Fluorometabolite biosynthesis: isotopically labelled glycerol incorporations into the antibiotic nucleocidin in Streptomyces calvus†
Abstract
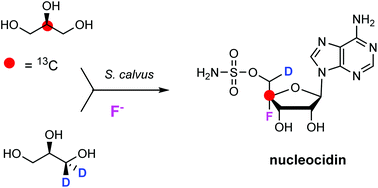
Deuterium and carbon-13 labelled glycerols have been fed to Streptomyces calvus fermentations and isotope incorporation into the fluorine containing antibiotic nucleocidin have been evaluated by 19F-NMR. A single deuterium atom was incorporated from [2H5]- and (R)-[2H2]-glycerol into C-5′ of the antibiotic, suggesting that an oxidation occurs at this carbon after ribose ring assembly from glycerol (pentose phosphate pathway), during nucleocidin biosynthesis.

 Please wait while we load your content...
Please wait while we load your content...