Oxoanion binding to a cyclic pseudopeptide containing 1,4-disubstituted 1,2,3-triazole moieties†
Abstract
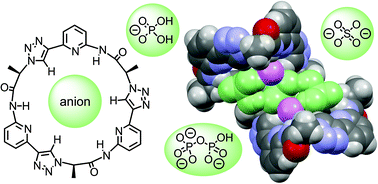
A macrocyclic pseudopeptide 3 is described featuring three amide groups and three 1,4-disubstituted 1,2,3-triazole units along the ring. This pseudopeptide was designed such that the amide NH groups and the triazole CH groups converge toward the cavity, thus creating an environment well suited for anion recognition. Conformational studies in solution combined with X-ray crystallography confirmed this preorganisation. Solubility of 3 restricted binding studies to organic media such as 5 vol% DMSO/acetone or DMSO/water mixtures with a water content up to 5 vol%. These binding studies demonstrated that 3 binds to a variety of inorganic anions in DMSO/acetone including chloride, nitrate, sulfate, and dihydrogenphosphate anions. In the more competitive DMSO/water mixtures, only affinity to the more strongly coordinating oxoanions is retained. Quantitative binding studies showed that dihydrogen phosphate complexation in DMSO/water involves the dimer of the H2PO4− anion. By contrast, sulfate and hydrogenpyrophosphate complexation involves a stepwise process comprising formation of a 1 : 1 complex followed by a 2R : 1A complex in which two molecules of 3 (R) bind to a single anion (A). While the second binding equilibrium is associated with a much smaller stability constant in comparison with the first one in the case of sulfate complexation, the two binding constants are of similar magnitude in the case of the hydrogenpyrophosphate anion. Formation of the 2R : 1A complex was attributed to the fact that the cavity size and rigidity of 3 prevents saturation of all hydrogen acceptor sites on the anionic guests.


 Please wait while we load your content...
Please wait while we load your content...