Enhancing the free corrosion dealloying rate with a catalytically driven reaction†
Abstract
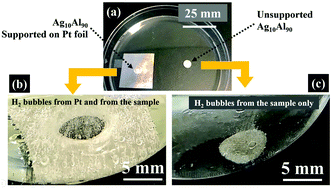
Despite its high popularity, chemical dealloying that is widely used for the fabrication of nanoporous metals is a relatively slow process: dealloying a few milligrams of bulk material may take from several hours up to a few days, depending on the material system. Raising the temperature of the corroding medium is a common approach to speed up the dealloying process. However, high temperatures cause undesired ligament growth in dealloyed materials. Here we report for the first time the use of a catalytically driven reaction to speed up the dealloying process at ambient temperature and pressure. To demonstrate the concept, we show that the free corrosion dealloying of a silver–aluminum alloy is significantly faster with the help of a platinum catalyst. More importantly, the corresponding characteristic nanostructured size is much smaller than that without a catalyst. Our finding is expected to play a central role in scaling up the dealloying process from the laboratory to the industrial scale.


 Please wait while we load your content...
Please wait while we load your content...