n- versus p-doping of graphite: what drives its wet-chemical exfoliation?†
Abstract
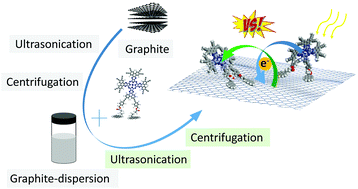
We have performed the syntheses of a novel pyrene-porphyrazine conjugate (ZnPzPy) and a reference porphyrazine (ZnPz) to promote the wet-chemical exfoliation of graphite based on the synergetic use of ultrasonication, centrifugation, and doping. ZnPzPy features, on one hand, a hydrophobic pyrene to anchor onto the basal plane of graphene, and, on the other hand, an amphoteric porphyrazine to either p- or n-dope graphene. To this end, we have characterized individual building blocks, that is, ZnPzPy and exfoliated graphite, and the resulting electron donor–acceptor nanohybrid, that is, ZnPzPy/graphene (ZnPzPy-G), by means of an arsenal of microscopic and spectroscopic techniques. From a full-fledged characterization we conclude that ZnPzPy facilitates the exfoliation of graphite affording suspensions featuring 9.5% of single- or few-layered ZnPzPy-G with a mean average size of 200 ± 140 nm. Importantly, a notable shift of charge density from graphene to ZnPzPy in the ground state of ZnPzPy-G corroborates the preference of exfoliated graphite to undergo p-doping rather than n-doping. As an immediate consequence, a full charge separation leads in the excited state to a 750 ± 150 ps lived charge separated state.


 Please wait while we load your content...
Please wait while we load your content...