Coordination of o-benzosemiquinonate, o-iminobenzosemiquinonate and aldimine anion radicals to oxidovanadium(iv)†
Abstract
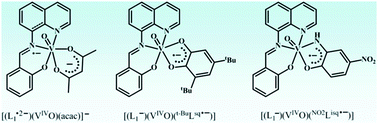
The study focuses on the stabilization of organic radicals by the oxidovanadium(IV) ion and it proves to be significant in exploring the bioactivity of vanadium. In addition to the o-benzosemiquinonate and o-iminobenzosemiquinonate anion radicals, the existence of reactive aldimine anion radicals coordinated to oxidovanadium(IV) ion was detected. Radical and non-radical oxidovanadium(IV) complexes of the types [(L1−)(VIVO)(acac)] (1), [(L2−)(VIVO)(acac)] (2), [(L1−)(VIVO)(sq˙−)] (3), [(L1−)(VIVO)(t-Busq˙−)] (4), [(L1−)(VIVO)(NO2isq˙−)] (5) and [(L1−)2(VIVO)2(SO4)]·½CH2Cl2 (6·½CH2Cl2) containing redox non-innocent tridentate NNO-donor aldimines (L1H and L2H) as coligands are reported (acac = acetylacetonato, sq˙− = o-benzosemiquinonate, t-Busq˙− = 3,5-di-tert-butyl-o-benzosemiquinonate and NO2isq˙− = p-nitro-o-iminobenzosemiquinonate anion radicals). The sq˙−, t-Busq˙− and NO2isq˙− states in complexes were established by X-ray crystallography, EPR spectroscopy and solid state cross polarization magic angle spinning (CP/MAS) 51V NMR spectroscopy, where the 51V nuclei in 3–5 were deshielded in a range from −100.3 to +608.7 ppm. The cathodic waves of 1 and 2 due to L1−/L1˙2− and L2−/L2˙2− redox couples are reversible. Density functional theory (DFT) calculations authenticated that 1− and 2− are open shell pi radical (L1˙2− and L2˙2−) complexes of oxidovanadium(IV). The energies of the open shell singlet (OSS) and triplet solutions of 1− and 2− are lower than the corresponding closed shell singlet (CSS) solutions. In 1− and 2− ions, 35–39% beta spin is localized on the πaldimine* function. The UV-vis-NIR absorption spectra of the complexes were analyzed by spectroelectrochemical measurements and time dependent (TD) DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...