Micro-engineered perfusable 3D vasculatures for cardiovascular diseases†
Abstract
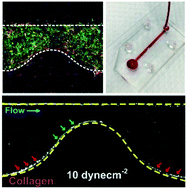
Vessel geometries in microengineered in vitro vascular models are important to recapitulate a pathophysiological microenvironment for the study of flow-induced endothelial dysfunction and inflammation in cardiovascular diseases. Herein, we present a simple and novel extracellular matrix (ECM) hydrogel patterning method to create perfusable vascularized microchannels of different geometries based on the concept of capillary burst valve (CBV). No surface modification is necessary and the method is suitable for different ECM types including collagen, matrigel and fibrin. We first created collagen-patterned, endothelialized microchannels to study barrier permeability and neutrophil transendothelial migration, followed by the development of a biomimetic 3D endothelial–smooth muscle cell (EC–SMC) vascular model. We observed a significant decrease in barrier permeability in the co-culture model during inflammation, which indicates the importance of perivascular cells in ECM remodeling. Finally, we engineered collagen-patterned constricted vascular microchannels to mimic stenosis in atherosclerosis. Whole blood was perfused (1–10 dyne cm−2) into the microdevices and distinct platelet and leukocyte adherence patterns were observed due to increased shear stresses at the constriction, and an additional convective flow through the collagen. Taken together, the developed hydrogel patterning technique enables the formation of unique pathophysiological architectures in organ-on-chip microsystems for real-time study of hemodynamics and cellular interactions in cardiovascular diseases.


 Please wait while we load your content...
Please wait while we load your content...