Dimethyldioxirane (DMDO) as a valuable oxidant for the synthesis of polyfunctional aromatic imidazolium monomers bearing epoxides†
Abstract
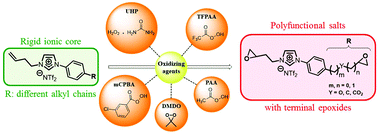
Conventional organic salts represent a new paradigm in many areas of research. Despite their great potential, an improvement in their physicochemical properties requires the chemical modification of their intrinsic structure. Thus, an efficient pathway was developed for the preparation of polyfunctional imidazolium monomers incorporating aromatic rings and terminal epoxides which presented a real synthetic challenge. In this work, we describe the reactivity of various oxidizing agents to develop a strong, clean and powerful methodology to generate epoxidized salts. Various reaction conditions for the formation of the epoxides were investigated such as the role of the cation and the counterion as well as the influence of an aromatic and/or aliphatic linker chain. Finally, we have evaluated the thermal properties of these new polyfunctional salts by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC).


 Please wait while we load your content...
Please wait while we load your content...