Recovery and esterification of aqueous carboxylates by using CO2-expanded alcohols with anion exchange†
Abstract
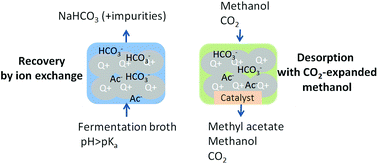
The recovery of carboxylic acids from fermentation broth is one of the main bottlenecks for the industrial production of bio-based esters. This paper proposes an alternative for the recovery of carboxylates produced by fermentations at pH values above the pKa of the carboxylic acid. In this approach, the aqueous carboxylate anion is recovered using anion exchange, followed by desorption and esterification with CO2-expanded alcohols. Using CO2-expanded methanol, we achieved a high desorption yield at 10 bar of CO2 and 20 °C. An ester yield of 1.03 ± 0.07 mol methyl acetate/acetatein was obtained for the combined desorption–esterification at 5 bar of CO2 and 60 °C. The proposed process has low chemical consumption and low waste production. The proposed process works, with a lower yield, for other carboxylates (e.g. lactate and succinate) and alcohols (e.g. ethanol).


 Please wait while we load your content...
Please wait while we load your content...