Photoassisted methanation using Cu2O nanoparticles supported on graphene as a photocatalyst†
Abstract
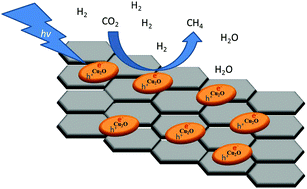
Photoassisted CO2 methanation can be carried out efficiently at 250 °C using Cu2O nanoparticles supported on few layer graphene (Cu2O/G) as a photocatalyst. The Cu2O/G photocatalyst has been prepared by chemical reduction of a Cu salt (Cu(NO3)2) with ethylene glycol in the presence of defective graphene obtained from the pyrolysis of alginic acid at 900 °C under Ar flow. Using this photocatalyst a maximum specific CH4 formation rate of 14.93 mmol gCu2O−1 h−1 and an apparent quantum yield of 7.84% were achieved, which are among the highest reported values for the gas-phase methanation reaction at temperatures below the Sabatier reaction temperature (>350 °C). It was found that the most probable reaction mechanism involves photoinduced electron transfer from the Cu2O/G photocatalyst to CO2, while evidence indicates that light-induced local temperature increase and H2 activation are negligible. The role of the temperature in the process has been studied, the available data suggesting that heating is needed to desorb the H2O formed as the product during the methanation. The most probable reaction mechanism seems to follow a dissociative pathway involving detachment of oxygen atoms from CO2.


 Please wait while we load your content...
Please wait while we load your content...