Cobalt, nickel, and iron complexes of 8-hydroxyquinoline-di(2-picolyl)amine for light-driven hydrogen evolution†
Abstract
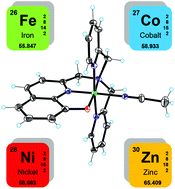
Novel cobalt, nickel, and iron complexes based on the pentadentate 8-hydroxyquinoline-di(2-picolyl)amine ligand were synthesized and thoroughly characterized. X-ray structures of both the cobalt and iron complexes were also obtained, showing the tendency to adopt a pseudo-octahedral geometry by coordination of an additional sixth ligand. These metal complexes were then studied as potential hydrogen-evolving catalysts (HECs) under both electrochemical and light-driven conditions. In particular, two different photochemical systems were tested involving either Ru(bpy)32+/ascorbic acid or Ir(ppy)2(bpy)+/TEA sensitizer/sacrificial donor couples. The electrochemical results showed that these metal complexes may behave as competent HECs. However, under photochemical conditions, only the cobalt compound displayed substantial hydrogen-evolving activity in both ruthenium- and iridium-based systems. The nickel and iron complexes, on the other hand, exhibited appreciable photocatalytic activity only in the iridium-based photochemical system, while showing negligible hydrogen evolution ability when employed in the ruthenium-based one.


 Please wait while we load your content...
Please wait while we load your content...