The impact of ionic liquids on the coordination of anions with solvatochromic copper complexes†
Abstract
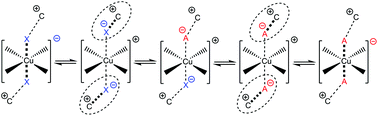
Solvatochromic transition metal (TM)-complexes with weakly associating counter-anions are often used to evaluate traditional neutral solvent and anion coordination ability. However, when employed in ionic liquids (IL) many of the common assumptions made are no longer reliable. This study investigates the coordinating ability of weakly coordinating IL anions in traditional solvents and within IL solvents employing a range of solvatochromic copper complexes. Complexes of the form [Cu(acac)(tmen)][X] (acac = acetylacetonate, tmen = tetramethylethylenediamine) where [X]− = [ClO4]−, Cl−, [NO3]−, [SCN]−, [OTf]−, [NTf2]− and [PF6]− have been synthesised and characterised both experimentally and computationally. ILs based on these anions and imidazolium and pyrrolidinium cations, some of which are functionalised with hydroxyl and nitrile groups, have been examined. IL-anion coordination has been investigated and compared to typical weakly coordinating anions. We have found there is potential for competition at the Cu-centre and cases of anions traditionally assigned as weakly associating that demonstrate a stronger than expected level of coordinating ability within ILs. [Cu(acac)(tmen)][PF6] is shown to contain the least coordinating anion and is established as the most sensitive probe studied here. Using this probe, the donor numbers (DNs) of ILs have been determined. Relative donor ability is further confirmed based on the UV-Vis of a neutral complex, [Cu(sacsac)2] (sacsac = dithioacetylacetone), and DNs evaluated via23Na NMR spectroscopy. We demonstrate that ILs can span a wide donor range, similar in breadth to conventional solvents.

 Please wait while we load your content...
Please wait while we load your content...