Half-sandwich iron(ii) complexes with protic acyclic diaminocarbene ligands: synthesis, deprotonation and metalation reactions†
Abstract
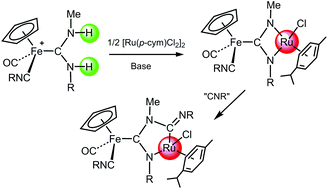
A variety of half-sandwich iron(II) complexes with diprotic acyclic diaminocarbene ligands (pADCs) have been obtained by reaction of the cationic complexes [Fe(Cp)(CO)2(CNR)]+ and [Fe(Cp)(CO)(CNR)2]+ with methylamine, and their acid–base behaviour was studied, revealing an easy reversible deprotonation reaction of both N–H moieties of the carbene ligands. The deprotonation process is frequently followed by a nucleophilic attack of the nitrogen atom on a vicinal carbonyl or isocyanide ligand, affording the corresponding metallacycles. Metalation of one or two N–H groups of the pADC ligands can be accomplished by reaction of the carbene complexes with either [AuCl(PPh3)] or [Ru(p-cym)Cl2]2 in the presence of KOH or LiHMDS as deprotonating agents. A number of Fe(II)/Au(I) and Fe(II)/Ru(II) heterometallic complexes have been prepared in this way, some of them formally containing unique metalla-N-heterocyclic carbene ligands.


 Please wait while we load your content...
Please wait while we load your content...