Kinetics of complexation of V(v), U(vi), and Fe(iii) with glutaroimide-dioxime: studies by stopped-flow and conventional absorption spectroscopy†
Abstract
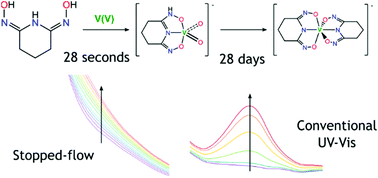
Uranium extracted from seawater is a promising source of uranium for nuclear energy, and the extraction technology using polymer sorbents has been shown to be feasible. However, improving selectivity for uranium over other metals, notably vanadium and iron, is essential to increase efficiency and reduce costs. In the present work, the kinetics of the binding of these three metals with glutaroimide-dioxime as a molecular analogue of polymer sorbents has been studied using stopped-flow and conventional UV Visible absorption spectroscopy to monitor the reactions over a range of time scales. Qualitatively, vanadium reacts the slowest of the three metals despite being able to form a very strong complex, with the 1 : 2 vanadium/ligand complex forming over weeks, likely due to the slow hydrolysis of the strong oxido ligands, while iron reacts fast and uranyl faster still, despite the presence of carbonate in the uranyl species. Conditional rate constants were determined for the formation of 1 : 1 glutaroimide-dioxime complexes with the three metal ions. Besides, in a narrow and near neutral pH region, a rate equation for the formation of the 1 : 1 vanadium/glutaroimide-dioxime complex was developed, showing the reaction is the first order with respect to [V], [ligand], and [H+]. These observations, some being qualitative and others quantitative, are consistent with previous marine tests of polymer adsorbents, and give mechanistic insight into how glutaroimide-dioxime forms complexes with uranium, iron, and vanadium.


 Please wait while we load your content...
Please wait while we load your content...