An iron germylene complex having Fe–H and Ge–H bonds: synthesis, structure and reactivity†
Abstract
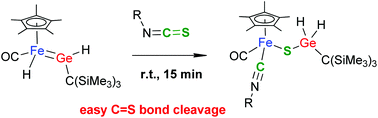
A base-free iron germylene complex Cp*(CO)(H)Fe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) GeH{C(SiMe3)3} (1) was synthesised by abstraction of pyridine from a germyl complex Cp*(CO)(py)FeGeH2{C(SiMe3)3} (2), which was prepared by treatment of Cp*Fe(CO)(py)(Me) with H3GeC(SiMe3)3. X-ray crystallographic analysis revealed that 1 has an Fe–Ge bond that is the shortest among those ever reported. Reactivity of 1 toward several polar unsaturated organic compounds was investigated. Complex 1 underwent stoichiometric hydrogermylation of carbonyl compounds such as ketones, aldehydes and isocyanates (RNCO) at room temperature. In contrast, the reactions of 1 with isothiocyanates (RNCS) resulted in clean cleavage of the C
GeH{C(SiMe3)3} (1) was synthesised by abstraction of pyridine from a germyl complex Cp*(CO)(py)FeGeH2{C(SiMe3)3} (2), which was prepared by treatment of Cp*Fe(CO)(py)(Me) with H3GeC(SiMe3)3. X-ray crystallographic analysis revealed that 1 has an Fe–Ge bond that is the shortest among those ever reported. Reactivity of 1 toward several polar unsaturated organic compounds was investigated. Complex 1 underwent stoichiometric hydrogermylation of carbonyl compounds such as ketones, aldehydes and isocyanates (RNCO) at room temperature. In contrast, the reactions of 1 with isothiocyanates (RNCS) resulted in clean cleavage of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S double bonds.
S double bonds.


 Please wait while we load your content...
Please wait while we load your content...