“Masked” Lewis-acidity of an aluminum α-phosphinoamide complex†
Abstract
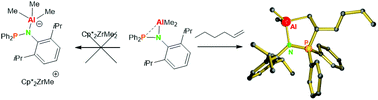
The reaction of Ph2P(DIPP)NH with AlMe3 cleanly gives an aluminum amide complex that crystallizes as a centrosymmetric dimer with a six-membered Al–N–P–Al–N–P ring. In aromatic solvents the dimer remains intact but the Al–P bond is readily broken upon addition of THF to form Ph2P(DIPP)NAlMe2·THF. Efforts to use [Ph2P(DIPP)NAlMe2]2 as a “masked” Lewis acidic activator for olefin polymerization catalysts were unsuccessful but the complex showed a Frustrated Lewis pair reactivity instead. The P/Al complex reacts with isocyanates to give the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O inserted product that crystallizes as a five-membered ring system Al–O–C(NR)–P–N. The reaction of [Ph2P(DIPP)NAlMe2]2 with CO2, however, gave an insertion in the N–Al bond and the dimeric product [Ph2P(DIPP)NCO2AlMe2]2 was isolated. The dimer [Ph2P(DIPP)NAlMe2]2 is one of the few Al/P FLPs that can activate C
O inserted product that crystallizes as a five-membered ring system Al–O–C(NR)–P–N. The reaction of [Ph2P(DIPP)NAlMe2]2 with CO2, however, gave an insertion in the N–Al bond and the dimeric product [Ph2P(DIPP)NCO2AlMe2]2 was isolated. The dimer [Ph2P(DIPP)NAlMe2]2 is one of the few Al/P FLPs that can activate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds irreversibly. A reaction with allyl methyl sulfide and 1-hexene led to the clean formation of the structurally similar activated alkene products [(DIPP)N-Ph2P-CH(CH2SMe)CH2]AlMe2 and [(DIPP)N-Ph2P-CH(C4H9)CH2]AlMe2.
C double bonds irreversibly. A reaction with allyl methyl sulfide and 1-hexene led to the clean formation of the structurally similar activated alkene products [(DIPP)N-Ph2P-CH(CH2SMe)CH2]AlMe2 and [(DIPP)N-Ph2P-CH(C4H9)CH2]AlMe2.


 Please wait while we load your content...
Please wait while we load your content...