3D oxalato-bridged lanthanide(iii) MOFs with magnetocaloric, magnetic and photoluminescence properties†
Abstract
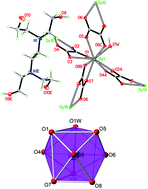
Four isostructural 3D lanthanide(III) metal–organic frameworks with the general formula (H6edte)0.5[LnIII(ox)2(H2O)] (Ln = Gd (1), Tb (2), Dy (3) and Ho (4); H4edte = N,N,N′,N′-tetrakis(2-hydroxyethyl)ethylenediamine) and ox = oxalate have been synthesized from oxalate. These compounds were characterized using single-crystal X-ray diffraction, FTIR and TGA/DTG. The magnetic investigation has been performed for 1–4 and the results reveal that a Gd(III) containing MOF has the highest magnetic density and exhibits a larger cryogenic magnetocaloric effect (ΔSm = 35.9 J kg−1 K−1 for ΔH = 9 T at 2.0 K) with a high Debye temperature (θD = 342(3) and rD = 35). For the Dy derivative, an ac-out-of-phase signal was found, and further measurements under an applied field of 1000 Oe showed frequency-dependent out-of-phase magnetic susceptibility. Solid-state luminescence indicates that Tb and Dy-containing complexes are photoluminescent materials.
- This article is part of the themed collection: Dalton Transactions Inorganic Symposia


 Please wait while we load your content...
Please wait while we load your content...