Stabilization and activation of unstable propynal in the zeolite nanospace and its application to addition reactions†
Abstract
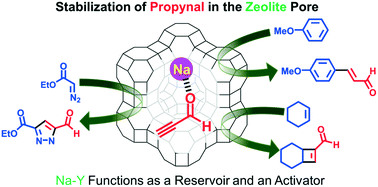
Propynal (HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CHO), having both a C
C–CHO), having both a C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C triple bond and a formyl group in a molecule, is a promising building block but its labile property to easily polymerize often narrows its application for organic synthesis. In a similar way to unstable molecules, such as formaldehyde and acrolein, propynal is also stabilized and remains unchanged in supercages of Na–Y zeolite for over 30 days at ambient temperature. There, the carbonyl oxygen atoms of propynal coordinate to sodium ions in Na–Y which was proved by a 13C-DD/MAS-NMR analysis. In addition, propynal adsorbed in zeolite is sufficiently activated to allow unprecedented reactions; i.e., (1) a 1,3-dipolar cycloaddition with electron-deficient α-diazocarbonyl compounds, (2) a 1,4-addition with mono-, di-, and trimethoxy-substituted benzenes, and (3) a [2 + 2] cycloaddition of unactivated cycloalkenes. The nanospace of the zeolites keeps the products from dimerization during reaction (1) and from successive side-reactions in reaction (2). Quantum chemical calculations demonstrated that reaction (3) proceeds via a one-step-like non-concerted mechanism to afford the corresponding [2 + 2] cycloadducts. These three reactions can produce valuable synthetic intermediates retaining both a formyl group and a C
C triple bond and a formyl group in a molecule, is a promising building block but its labile property to easily polymerize often narrows its application for organic synthesis. In a similar way to unstable molecules, such as formaldehyde and acrolein, propynal is also stabilized and remains unchanged in supercages of Na–Y zeolite for over 30 days at ambient temperature. There, the carbonyl oxygen atoms of propynal coordinate to sodium ions in Na–Y which was proved by a 13C-DD/MAS-NMR analysis. In addition, propynal adsorbed in zeolite is sufficiently activated to allow unprecedented reactions; i.e., (1) a 1,3-dipolar cycloaddition with electron-deficient α-diazocarbonyl compounds, (2) a 1,4-addition with mono-, di-, and trimethoxy-substituted benzenes, and (3) a [2 + 2] cycloaddition of unactivated cycloalkenes. The nanospace of the zeolites keeps the products from dimerization during reaction (1) and from successive side-reactions in reaction (2). Quantum chemical calculations demonstrated that reaction (3) proceeds via a one-step-like non-concerted mechanism to afford the corresponding [2 + 2] cycloadducts. These three reactions can produce valuable synthetic intermediates retaining both a formyl group and a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond.
C double bond.


 Please wait while we load your content...
Please wait while we load your content...