A comparative study of hydrogen evolution reaction on pseudo-monolayer WS2 and PtS2: insights based on the density functional theory†
Abstract
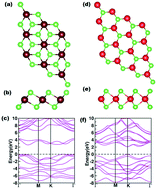
In this study, we investigated the catalytic activity of ultrathin PtS2 and WS2 nanostructures for the hydrogen evolution reaction by electronic structure calculations based on the spin-polarised density functional theory. We also explored the effect of van der Waals interactions on the surface–adsorbate interactions. Using the adsorption free energy of H2 as an activity descriptor, we tuned the photocatalytic water splitting activity of PtS2 and WS2 by functionalizing the individual systems with different transition metals such as Ru, Rh, Pd, Ag, Ir, Au, and Hg. The density of states was calculated along with the band structure to find the effect of different dopants on the fundamental band gap, which is one of the primary parameters in the photocatalytic water splitting.


 Please wait while we load your content...
Please wait while we load your content...