The mechanism of H2 and H2O desorption from bridging hydroxyls of a TiO2(110) surface†
Abstract
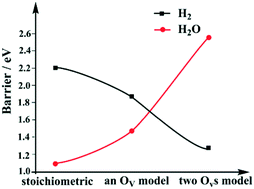
The photocatalytic H2 production from H2O over TiO2 has attracted tremendous attention in recent years, and great progress has been achieved in light adsorption and water dissociation. As a comparison, the H2 and O2 production over a pure TiO2 surface has not been successful yet. Recently, the desorption of bridging hydroxyls (BBOHs) to form H2 has been found to be possible on a TiO2(110) surface. However, the yield of H2 is low, and the majority of BBOHs desorb as H2O. Here, for the first time, we have systematically studied the mechanism of H2 desorption and the competition of H2 and H2O desorption on a TiO2(110) surface with DFT methods. We found that the generally believed pathway, the direct coupling of BBOHs, is not the most facile pathway. We propose that the reaction undergoes a Ti–H key intermediate, which then forms an O–Hδ+⋯Hδ−–Ti type dehydrogenation transition state. On a stoichiometric surface, the barriers for H2 desorption and H2O desorption are 2.20 eV and 1.09 eV, respectively. However, with an increase in BBO vacancies (Ovs, created by H2O desorption), the barrier for H2 desorption decreases, while the barrier for H2O desorption increases. Specifically, H2 desorption was found to be easier than H2O desorption with our two Ovs TiO2 model. Therefore, our results predict that H2O desorption is dominant at the beginning, then H2 desorption becomes more and more competitive. This prediction perfectly fits with the experimental observations. Furthermore, the stability of the key Ti–H intermediates and the possible ways for changing the conditions of H2 desorption have also been discussed.

 Please wait while we load your content...
Please wait while we load your content...