Nanoparticles grown from methanesulfonic acid and methylamine: microscopic structures and formation mechanism†
Abstract
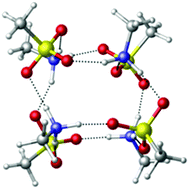
Mechanisms of particle formation and growth in the atmosphere are of great interest due to their impacts on climate, health and visibility. However, the microscopic structures and related properties of the smallest nanoparticles are not known. In this paper we pursue computationally a microscopic description for the formation and growth of methanesulfonic acid (MSA) and methylamine (MA) particles under dry conditions. Energetic and dynamics simulations were used to assess the stabilities of proposed model structures for these particles. Density functional theory (DFT) and semi-empirical (PM3) calculations suggest that (MSA–MA)4 is a major intermediate in the growth process, with the dissociation energies, enthalpies and free energies indicating considerable stability for this cluster. Dynamics simulations show that this species is stable for at least 100 ps at temperatures up to 500 K, well above atmospheric temperatures. In order to reach experimentally detectable sizes (>1.4 nm), continuing growth is suggested to occur via clustering of (MSA–MA)4. The dimer (MSA–MA)4⋯(MSA–MA)4 may be one of the smaller experimentally measured particles. Step by step addition of MSA to (MSA–MA)4, is also a likely potential growth mechanism when MSA is excess. In addition, an MSA–MA crystal is predicted to exist. These studies demonstrate that computations of particle structure and dynamics in the nano-size range can be useful for molecular level understanding of processes that grow clusters into detectable particles.


 Please wait while we load your content...
Please wait while we load your content...