UV-Vis spectrophotometry of quinone flow battery electrolyte for in situ monitoring and improved electrochemical modeling of potential and quinhydrone formation†
Abstract
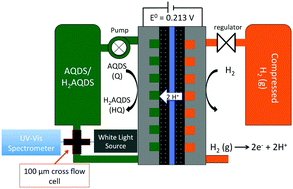
Quinone-based aqueous flow batteries provide a potential opportunity for large-scale, low-cost energy storage due to their composition from earth abundant elements, high aqueous solubility, reversible redox kinetics and their chemical tunability such as reduction potential. In an operating flow battery utilizing 9,10-anthraquinone-2,7-disulfonic acid, the aggregation of an oxidized quinone and a reduced hydroquinone to form a quinhydrone dimer causes significant variations from ideal solution behavior and of optical absorption from the Beer–Lambert law. We utilize in situ UV-Vis spectrophotometry to establish (a), quinone, hydroquinone and quinhydrone molar attenuation profiles and (b), an equilibrium constant for formation of the quinhydrone dimer (KQHQ) ∼ 80 M−1. We use the molar optical attenuation profiles to identify the total molecular concentration and state of charge at arbitrary mixtures of quinone and hydroquinone. We report density functional theory calculations to support the quinhydrone UV-Vis measurements and to provide insight into the dimerization conformations. We instrument a quinone–bromine flow battery with a Pd–H reference electrode in order to demonstrate how complexation in both the negative (quinone) and positive (bromine) electrolytes directly impacts measured half-cell and full-cell voltages. This work shows how accounting for electrolyte complexation improves the accuracy of electrochemical modeling of flow battery electrolytes.


 Please wait while we load your content...
Please wait while we load your content...