Voltammetric and spectroscopic study of ferrocene and hexacyanoferrate and the suitability of their redox couples as internal standards in ionic liquids†
Abstract
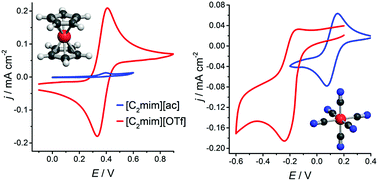
Ionic liquids and deep eutectic solvents have great potential in metallurgical applications as specialised solvents. In order to design ionometallurgical electrowinning and electrorefining processes, it is essential to characterise the electrochemical behaviour of metal complexes and compare potentials between relevant solvents. For such investigations, a universal reference redox couple would be desirable. In this study we investigate the speciation and electrochemical behaviour of ferrocenium/ferrocene and hexacyanoferrate(III/II) as possible reference couples for 15 different ionic media on platinum (Pt), glassy carbon (GC) and gold (Au) working electrodes. Amongst other parameters, formal electrode potentials, charge transfer coefficients, and rate constants were calculated. It was found that neither ferrocene nor hexacyanoferrate are universally suitable as redox standards in the liquids investigated. Nevertheless, hexacyanoferrate exhibits clear advantages in most of the strongly coordinating ionic liquids studied here.


 Please wait while we load your content...
Please wait while we load your content...