Augmented pH-sensitivity absorbance of a ruthenium(ii) bis(bipyridine) complex with elongation of the conjugated ligands: an experimental and theoretical investigation
Abstract
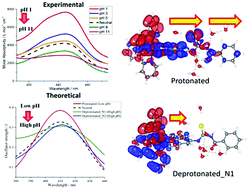
An absorbance-based sensor employing ruthenium bipyridyl with a phenanthroline-fused benzoylthiourea moiety formulated as [Ru(II)(bpy)2(phen-nBT)](PF6)2 {bpy = 2,2′-bipyridine, phen = 1,10-phenanthroline, nBT = n-benzoylthiourea} has been synthesized and characterized by elemental analyses, mass spectrometry, and infrared, ultraviolet-visible, luminescence and nuclear magnetic resonance spectroscopy. The changes in the intensity of absorption and emission of the complex induced by functionalization of the benzoylthiourea ligands with amino and carbonyl in their protonated and deprotonated forms were studied experimentally. The absorption and emission properties of the complex exhibit a strong dependence on the pH (1–11) of the aqueous medium. This work highlights the pH-sensitivity augmentation of the absorption band by elongating the conjugation length in the structure of the ruthenium bipyridine complex. The principle of this work was to design the title compound to be capable of enhancing the differences in the absorption sensitivity responses towards pH between the protonated and deprotonated complexes in the absorption measurement. Along with significant and noticeable changes in the absorption spectra, subsequent theoretical investigations specifically on the electronic and absorbance properties of the title compound were carried out in this study. Protonation of the molecule significantly stabilized the lowest-unoccupied molecular orbital (LUMO), whereas the highest-occupied molecular orbital (HOMO) is greatly destabilized upon deprotonation. A time-dependent density functional theory (TDDFT) calculation in the linear-response (-LR) regime was performed to clarify the origin of the experimentally observed linear dependence of absorption intensity upon pH (1–11). The MLCT band exhibits hyperchromic shift at low pH as indicated by the large transition dipole moment and a wider distribution of the response charge of the molecule, which is induced by the stabilization of the electrostatic potential at the carbonyl moiety by protonation. This study provides the possibility of employing theoretical information to gain insight into the origin of the optical absorption obtained experimentally. The ruthenium complex was designed with an elongated ligand conjugation length and exhibited a tremendously large change in the absorption intensity of the protonated and deprotonated forms, which therefore demonstrates its feasibility as an indicator molecule especially for absorbance measurements.


 Please wait while we load your content...
Please wait while we load your content...