Influence of co-non-solvency on hydrophobic molecules driven by excluded volume effect
Abstract
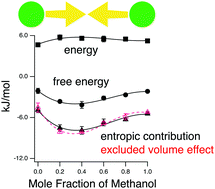
We demonstrate by molecular dynamics simulation that co-non-solvency manifests itself in the solvent-induced interaction between three hydrophobes, methane, propane and neopentane, in methanol–water mixtures. Decomposition of the potential of mean force, based on the potential distribution theorem, clearly shows that the solute–solvent entropic change is responsible for stabilizing the aggregation of these hydrophobic molecules. Furthermore, we show that the entropic change pertains to the excluded volume effect.


 Please wait while we load your content...
Please wait while we load your content...