Characterization of hydrogen bonding motifs in proteins: hydrogen elimination monitoring by ultraviolet photodissociation mass spectrometry†
Abstract
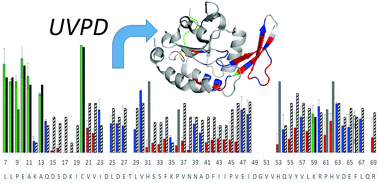
Determination of structure and folding of certain classes of proteins remains intractable by conventional structural characterization strategies and has spurred the development of alternative methodologies. Mass spectrometry-based approaches have a unique capacity to differentiate protein heterogeneity due to the ability to discriminate populations, whether minor or major, featuring modifications or complexation with non-covalent ligands on the basis of m/z. Cleavage of the peptide backbone can be further utilized to obtain residue-specific structural information. Here, hydrogen elimination monitoring (HEM) upon ultraviolet photodissociation (UVPD) of proteins transferred to the gas phase via nativespray ionization is introduced as an innovative approach to deduce backbone hydrogen bonding patterns. Using well-characterized peptides and a series of proteins, prediction of the engagement of the amide carbonyl oxygen of the protein backbone in hydrogen bonding using UVPD-HEM is demonstrated to show significant agreement with the hydrogen-bonding motifs derived from molecular dynamics simulations and X-ray crystal structures.


 Please wait while we load your content...
Please wait while we load your content...