Low-lying, Rydberg states of polycyclic aromatic hydrocarbons (PAHs) and cyclic alkanes†
Abstract
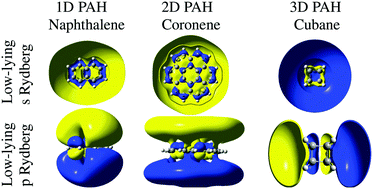
TD-DFT calculations of low-lying, Rydberg states of a series of polycyclic hydrocarbons and cyclic alkanes are presented. Systematic variations in binding energies and photoelectron angular distributions for the first members of the s, p and d Rydberg series are predicted for increasing molecular complexity. Calculated binding energies are found to be in very good agreement with literature values where they exist for comparison. Experimental angle-resolved photoelectron spectroscopy results are presented for coronene, again showing very good agreement with theoretical predictions of binding energies and also for photoelectron angular distributions. The Dyson orbitals for the small “hollow” carbon structures, cubane, adamantane and dodecahedrane, are shown to have close similarities to atomic s, p and d orbitals, similar to the superatom molecular orbitals (SAMOs) reported for fullerenes, indicating that these low-lying, diffuse states are not restricted to π-conjugated molecules.


 Please wait while we load your content...
Please wait while we load your content...