Ionic liquids for metal extraction from chalcopyrite: solid, liquid and gas phase studies†
Abstract
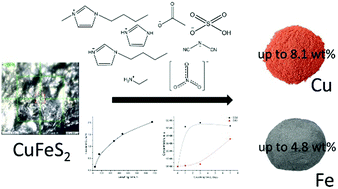
We studied leaching of Cu and Fe from naturally occurring chalcopyrite ore using aqueous solutions of ionic liquids (ILs) based on imidazolium and ethylammonium cations and hydrogensulfate, nitrate, acetate or dicyanamide anions. Liquid, solid and gas phases of the leaching systems were characterised. We have shown that nonoxidative leaching is greatly dependant not only on temperature and pH, but on the anion species of the IL. Solutions of 1-butylimidazolium hydrogen sulfate exhibited the best leaching performance among hydrogen sulphate ILs. We have suggested that the formation of an oxide layer in some ILs may be responsible for a reduced leaching ability. The analysis of the gas phase showed the production of CO2 and CS2 in all leached samples. Our results suggested that the CS2 produced upon leaching could be responsible for decreasing the sulfur, but not oxide, layer on the surface of chalcopyrite samples and therefore more efficient leaching. This is the first study, to our knowledge, to provide a systematic comparison of the leaching performance of ILs composed of different anions and cations and without added oxidants.


 Please wait while we load your content...
Please wait while we load your content...