Fast crystalline ice formation at extremely low temperature through water/neon matrix sublimation†
Abstract
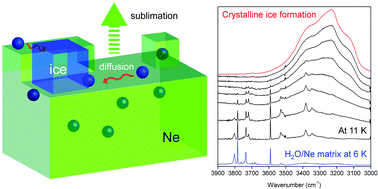
Crystalline ice formation requires water molecules to be sufficiently mobile to find and settle on the thermodynamically most stable site. Upon cooling, however, diffusion and rearrangement become increasingly kinetically difficult. Water ice grown by the condensation of water vapor in laboratory is thus generally assumed to be in a metastable amorphous form below 100 K. Here, we demonstrate the possibility of crystalline ice formation at extremely low temperature using a water/neon matrix (1/1000, 30 000 monolayers) prepared at 6 K, which is subsequently warmed to 11–12 K. In situ infrared spectroscopy revealed the assembly of the dispersed water molecules, forming crystalline ice I during the sublimation of the neon matrix for 40–250 seconds. This finding indicates that the high mobility of the water molecules during matrix sublimation can overcome the kinetic barrier to form crystals even at extremely low temperature.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...