Direct examination of the relevance for folding, binding and electron transfer of a conserved protein folding intermediate†
Abstract
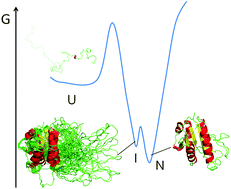
Near the minimum free energy basin of proteins where the native ensemble resides, partly unfolded conformations of slightly higher energy can be significantly populated under native conditions. It has been speculated that they play roles in molecular recognition and catalysis, but they might represent contemporary features of the evolutionary process without functional relevance. Obtaining conclusive evidence on these alternatives is difficult because it requires comparing the performance of a given protein when populating and when not populating one such intermediate, in otherwise identical conditions. Wild type apoflavodoxin populates under native conditions a partly unfolded conformation (10% of molecules) whose unstructured region includes the binding sites for the FMN cofactor and for redox partner proteins. We recently engineered a thermostable variant where the intermediate is no longer detectable. Using the wild type and variant, we assess the relevance of the intermediate comparing folding kinetics, cofactor binding kinetics, cofactor affinity, X-ray structure, intrinsic dynamics, redox potential of the apoflavodoxin–cofactor complex (Fld), its affinity for partner protein FNR, and electron transfer rate within the Fld/FNR physiological complex. Our data strongly suggest the intermediate state, conserved in long-chain apoflavodoxins, is not required for the correct assembly of flavodoxin nor does it contribute to shape its electron transfer properties. This analysis can be applied to evaluate other native basin intermediates.


 Please wait while we load your content...
Please wait while we load your content...