Rates and equilibrium constants of the ligand-induced conformational transition of an HCN ion channel protein domain determined by DEER spectroscopy†
Abstract
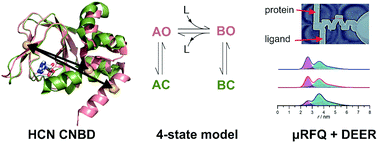
Ligand binding can induce significant conformational changes in proteins. The mechanism of this process couples equilibria associated with the ligand binding event and the conformational change. Here we show that by combining the application of W-band double electron–electron resonance (DEER) spectroscopy with microfluidic rapid freeze quench (μRFQ) it is possible to resolve these processes and obtain both equilibrium constants and reaction rates. We studied the conformational transition of the nitroxide labeled, isolated carboxy-terminal cyclic-nucleotide binding domain (CNBD) of the HCN2 ion channel upon binding of the ligand 3′,5′-cyclic adenosine monophosphate (cAMP). Using model-based global analysis, the time-resolved data of the μRFQ DEER experiments directly provide fractional populations of the open and closed conformations as a function of time. We modeled the ligand-induced conformational change in the protein using a four-state model: apo/open (AO), apo/closed (AC), bound/open (BO), bound/closed (BC). These species interconvert according to AC + L ⇌ AO + L ⇌ BO ⇌ BC. By analyzing the concentration dependence of the relative contributions of the closed and open conformations at equilibrium, we estimated the equilibrium constants for the two conformational equilibria and the open-state ligand dissociation constant. Analysis of the time-resolved μRFQ DEER data gave estimates for the intrinsic rates of ligand binding and unbinding as well as the rates of the conformational change. This demonstrates that DEER can quantitatively resolve both the thermodynamics and the kinetics of ligand binding and the associated conformational change.


 Please wait while we load your content...
Please wait while we load your content...