Charge-transfer states in triazole linked donor–acceptor materials: strong effects of chemical modification and solvation†
Abstract
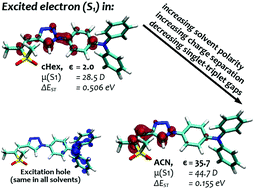
A series of 1,2,3-triazole linked donor–acceptor chromophores are prepared by Click Chemistry from ene–yne starting materials. The effects of three distinct chemical variations are investigated: enhancing the acceptor strength through oxidation of the sulphur atom, alteration of the double bond configuration, and variation of the triazole substitution pattern. A detailed photophysical characterization shows that these alterations have a negligible effect on the absorption while dramatically altering the emission wavelengths. In addition, strong solvatochromism is found leading to significant red shifts in the case of polar solvents. The experimental findings are rationalized and related to the electronic structure properties of the chromophores by time-dependent density functional theory as well as the ab initio algebraic diagrammatic construction method for the polarization propagator in connection with a new formalism allowing to model the influence of solvation onto long-lived excited states and their emission energies. These calculations highlight the varying degree of intramolecular charge transfer character present for the different molecules and show that the amount of charge transfer is strongly modulated by the conducted chemical modifications, by the solvation of the chromophores, and by the structural relaxation in the excited state. It is, furthermore, shown that enhanced charge separation, as induced by chemical modification or solvation, reduces the singlet–triplet gaps and that two of the investigated molecules possess sufficiently low gaps to be considered as candidates for thermally activated delayed fluorescence.


 Please wait while we load your content...
Please wait while we load your content...