Surface-induced assembly of sophorolipids†
Abstract
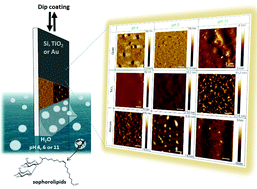
The surface self-assembly properties of acidic sophorolipids, a bolaform microbial glycolipids with pH-responsive properties in solution, were studied based on the chemical nature of the support and pH of the solution. Sophorolipids generally form micelles in water but formation of morphologies like platelets and twisted fibers depending on pH have also been reported. The surface self-assembly was achieved using dip-coating on three different substrates namely gold, silicon(111) and TiO2 anatase. Deposition conditions (dip-coating withdrawal speed, relative humidity, temperature) were tested, and it was found that optimum self-assembly occurs at a withdrawal speed of 1 mm s−1, T of 25 °C and relative humidity of 25%. The local structure of the sophorolipid films was characterized by atomic force microscopy, while scanning electron microscopy was used to characterize the spatial homogeneity. We also attempted to correlate dispersive, electron donor and electron attractor surface energy components, using Good–van Oss's approach, and the behavior of sophorolipids. We found that when the surface energy is dominated by dispersive components, sophorolipids spontaneously assemble into entangled needles at all pH values (4, 6 and 11). However, when the surface energy is dominated by electronic components, pH has a strong influence on the surface self-assembly. We could discriminate three major organizations: homogeneous layer, isolated aggregates and a two-dimensional fibrillar network.


 Please wait while we load your content...
Please wait while we load your content...