Interfacial interactions between CoTPP molecules and MgO(100) thin films†
Abstract
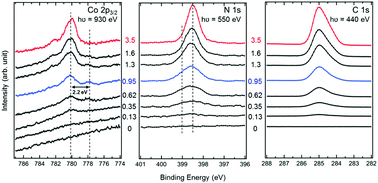
We have investigated the interactions between cobalt(II)-tetraphenylporphyrin (CoTPP) molecules and MgO(100) thin films on Ag(100) by means of Synchrotron Radiation X-Ray and Ultra-Violet Photoelectron Spectroscopy (SR-XPS and SR-UPS). At room temperature, the CoTPP monolayer consists of two different species. A minority of molecules exhibits a strong electronic interaction with the substrate, whereas for the majority a similar spectroscopic signature as for multilayer molecules is observed. Based on the lateral inhomogeneity of the surface electronic structure, we tentatively suggest that the strongly interacting molecules adsorb with their metal center directly above oxygen ions. Unlike for metal substrates, where a monolayer can be prepared upon heating to above 500 K, most of the monolayer on MgO desorbs at 550 K together with the multilayers. This indicates either a weaker molecule–substrate bond than for most metal surfaces or a higher activation energy barrier for dehydrogenation. The remaining molecules are presumably MgTPP molecules, originating from a 2HTPP impurity in CoTPP.


 Please wait while we load your content...
Please wait while we load your content...