Electrical anharmonicity in hydrogen bonded systems: complete interpretation of the IR spectra of the Cl–![[H with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/char_0048_20d1.gif) stretching band in the gaseous (CH3)2O⋯HCl complex†
stretching band in the gaseous (CH3)2O⋯HCl complex†
Abstract
Following the previous developments to simulate the fully infrared spectra of weak hydrogen bond systems within the linear response theory, an extension of the adiabatic model is presented here. A general formulation including the electrical anharmonicities in the calculation of the damped autocorrelation function of weak H-bonds is adopted to facilitate the support of the additional properties, and thus the IR spectra of the Cl–![[H with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/char_0048_20d1.gif) stretching band in the gaseous (CH3)2O⋯HCl complex. We have explored the origins of the broadening of the Cl–
stretching band in the gaseous (CH3)2O⋯HCl complex. We have explored the origins of the broadening of the Cl–![[H with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/char_0048_20d1.gif) stretching band. We found that the main features of the lineshape are attributed to electrical anharmonicity as a consequence of the large mixed second derivatives of the dipole moment with respect to the Cl–
stretching band. We found that the main features of the lineshape are attributed to electrical anharmonicity as a consequence of the large mixed second derivatives of the dipole moment with respect to the Cl–![[H with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/char_0048_20d1.gif) bond and of the intermonomer elongations
bond and of the intermonomer elongations 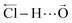 . In addition to providing more accurate theoretical band shapes, inclusion of the electrical anharmonicity in the present model paves the way for a more complete interpretation by generating three new Franck–Condon superposed distributions.
. In addition to providing more accurate theoretical band shapes, inclusion of the electrical anharmonicity in the present model paves the way for a more complete interpretation by generating three new Franck–Condon superposed distributions.
![Graphical abstract: Electrical anharmonicity in hydrogen bonded systems: complete interpretation of the IR spectra of the Cl– [[H with combining right harpoon above (vector)]] stretching band in the gaseous (CH3)2O⋯HCl complex](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C7CP00165G&imageInfo.ImageIdentifier.Year=2017)


 Please wait while we load your content...
Please wait while we load your content...
![[H with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/h2_char_0048_20d1.gif) stretching band in the gaseous (CH3)2O⋯HCl complex
stretching band in the gaseous (CH3)2O⋯HCl complex