Incorporation of vanadium into the framework of hydroxyapatites: importance of the vanadium content and pH conditions during the precipitation step†
Abstract
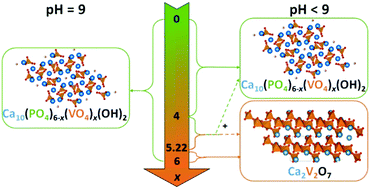
Even though vanadium-modified hydroxyapatite (V-HAp) samples are very promising systems for oxidative dehydrogenation of propane, the incorporation of vanadium into the hydroxyapatite framework was reported to be limited and to lead to over-stoichiometric compounds. Here, the synthesis of a Ca10(PO4)6−x(VO4)x(OH)2 stoichiometric solid solution using a co-precipitation method is monitored in the whole composition range (0 ≤ x ≤ 6) by controlling the pH of the precipitation medium, with continuous (the first series of samples) or periodic (the second series of samples) addition of NH4OH during the precipitation step or during the maturation step, respectively. It is demonstrated that the changes in pH conditions result in materials of a substantial difference in terms of the final composition. From XRD patterns and Rietveld refinements, a solid solution V-HAp phase was found to be exclusively obtained for the first series of samples for x varying from 0 to 6. This also occurred in the second series of samples but only for x lower than 4. For 4 ≤ x ≤ 5.22, the materials were composed of a mixture of V-HAp and Ca2V2O7, whereas for a x value of 6 only Ca2V2O7 was formed. The predominance of polymeric V species in solution at a high vanadium concentration deduced from the diagram of speciation of vanadium accounts for the preferential formation of Ca2V2O7 under these particular conditions. However, provided that a higher pH value was maintained, isolated VO3(OH)2− species are predominant, which accounts for the incorporation of isolated vanadates into the hydroxyapatite framework and for the well-controlled stoichiometry with Ca/(P + V) ratios found to be close to 1.67. Such a very good accommodation of vanadium in the hydroxyapatite framework is illustrated by the characterization of the local surrounding of phosphorus and vanadium species using 31P and 51V NMR, Raman and UV-vis spectroscopies.


 Please wait while we load your content...
Please wait while we load your content...