Influence of the excitation light intensity on the rate of fluorescence quenching reactions: pulsed experiments
Abstract
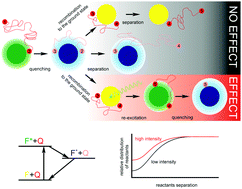
The effect of multiple light excitation events on bimolecular photo-induced electron transfer reactions in liquid solution is studied experimentally. It is found that the decay of fluorescence can be up to 25% faster if a second photon is absorbed after a first cycle of quenching and recombination. A theoretical model is presented which ascribes this effect to the enrichment of the concentration of quenchers in the immediate vicinity of fluorophores that have been previously excited. Despite its simplicity, the model delivers a qualitative agreement with the observed experimental trends. The original theory by Burshtein and Igoshin (J. Chem. Phys., 2000, 112, 10930–10940) was created for continuous light excitation though. A qualitative extrapolation from the here presented pulse experiments to the continuous excitation conditions lead us to conclude that in the latter the order of magnitude of the increase of the quenching efficiency upon increasing the light intensity of excitation, must also be on the order of tens of percent. These results mean that the rate constant for photo-induced bimolecular reactions depends not only on the usual known factors, such as temperature, viscosity and other properties of the medium, but also on the intensity of the excitation light.


 Please wait while we load your content...
Please wait while we load your content...