Trapping of excess energy in a nano-layered microenvironment to promote chemical reactions
Abstract
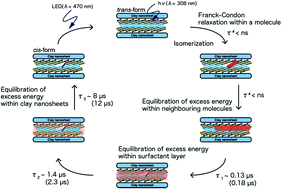
Nano-layered hybrid compounds composed of a polyfluoroalkyl azobenzene surfactant (abbreviated as C3F-Azo-C6H) and layered inorganic nanosheets undergo three-dimensional morphological changes such as reversible shrinkage and expansion of interlayer spaces, and nanosheet sliding by photo-irradiation. Previously, we have investigated the photoreactivity of C3F-Azo-C6H/clay nano-layered hybrids in various microenvironments and found a remarkable enhancement in the photoreactivity for the cis–trans photo-isomerization reaction (Φcis–trans = 1.9). In this paper, nanosecond and microsecond dynamics of trans-C3F-Azo-C6H and its assembly in various microenvironments have been studied by laser flash photolysis to get deeper insight into the extraordinary reactivity of the molecular assembly in the nano-layered microenvironment. In solution, the molecular trans-C3F-Azo-C6H exhibited only a depletion of the trans-form of azobenzene upon the laser pulse excitation. On the other hand, in the case of the C3F-Azo-C6H/clay hybrid film, the depletion of the trans-form was drastically recovered in three steps on nano- and microsecond timescales. This indicates that the once reacted C3F-Azo-C6H molecule (cis-C3F-Azo-C6H) was reverted back to the trans-form after the laser pulse. It is considered that the excess energy provided by the photo-excitation, which is immediately dissipated to the surrounding media through the intermolecular vibrational modes in solution, is trapped in the nano-layered microenvironment to thermally revert the cis-form back to the trans-form. Conversely, in the case of cis–trans isomerization of the C3F-Azo-C6H/clay hybrid film upon photo-irradiation, the reactivity would be much enhanced by the additional contribution of the thermal excess energy efficiently trapped in the nano-layered microenvironment. As compared with the hydrocarbon analogue (C3H-Azo-C6H), the subsequent recovery was very much enhanced in the C3F-Azo-C6H/clay film. The polyfluoroalkyl part of the surfactant layer plays a key role in the retarded dissipation of the excess energy by photo-excitation, which might be coupled with the three-dimensional morphological motion with efficient isomerization reactions.


 Please wait while we load your content...
Please wait while we load your content...