Detection of a higher energy isomer of the CO–N2O van der Waals complex and determination of two of its intermolecular frequencies†
Abstract
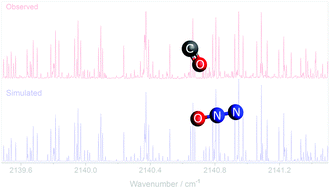
Infrared spectra in the carbon monoxide CO stretch region (∼2150 cm−1) and in the ν3 asymmetric stretch region of N2O (∼2223 cm−1) are assigned to the previously unobserved O-bonded form of the CO–N2O dimer (“isomer 2”). This van der Waals complex has a planar skewed T-shaped structure like that of the previously observed C-bonded form (“isomer 1”), but with the CO rotated by 180°. The effective intermolecular distance between the centers of mass is 3.51 Å for isomer 2 as compared to 3.88 Å for isomer 1. In addition to the fundamental band, two combination bands are observed for isomer 2, yielding values for two intermolecular vibrational modes: 14.502(5) cm−1 for the coupled disrotatory motion or the uncoupled CO rock and 21.219(5) cm−1 for the out-of-plane rock. We show that the published ab initio study on this system is inadequate in predicting the intermolecular frequencies for isomer 2 of CO–N2O.


 Please wait while we load your content...
Please wait while we load your content...