Theoretical study on substituent and solvent effects for nanocubes formed with gear-shaped amphiphile molecules†
Abstract
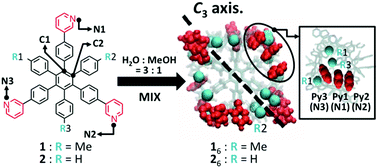
Gear-shaped amphiphile molecules (1) recently synthesized by Hiraoka et al. self-assemble into a hexameric structure, nanocubes (16), in 25% aqueous methanol due to a solvophobic effect. Here we have carried out molecular dynamic simulations to elucidate the stability of these hexameric capsules (16 and 26) in water, 25% aqueous methanol, and methanol. In all solvents, the 16 nanocubes are maintained for all trajectories. On the other hand, 26 was found to collapse for one trajectory in water and seven trajectories in 25% aqueous methanol. In a pure methanol solvent, 26 was found to collapse for all trajectories. The number of collapsed trajectories of 26 increased with the amount of methanol in the solvent. We therefore focused on the structure of the π–π stacking between pyridyl groups and the CH–π interactions between the methyl and pyridyl groups within the nanocube. Our study clearly shows the role played by the methanol solvent molecules in the assembly of the nanocube in terms of the substituent and solvent effects at the molecular level, and that these substituent and solvent effects are important for the self-assembly of the nanocubes.

 Please wait while we load your content...
Please wait while we load your content...