Reactive collisions for NO(2Π) + N(4S) at temperatures relevant to the hypersonic flight regime
Abstract
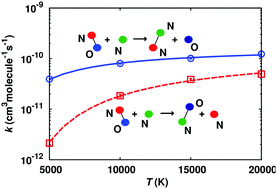
The NO(X2Π) + N(4S) reaction which occurs entirely in the triplet manifold of N2O is investigated using quasiclassical trajectories and quantum simulations. Fully-dimensional potential energy surfaces for the 3A′ and 3A′′ states are computed at the MRCI+Q level of theory and are represented using a reproducing kernel Hilbert space. The N-exchange and N2-formation channels are followed by using the multi-state adiabatic reactive molecular dynamics method. Up to 5000 K these reactions occur predominantly on the N2O 3A′′ surface. However, for higher temperatures the contributions of the 3A′ and 3A′′ states are comparable and the final state distributions are far from thermal equilibrium. From the trajectory simulations a new set of thermal rate coefficients of up to 20 000 K is determined. Comparison of the quasiclassical trajectory and quantum simulations shows that a classical description is a good approximation as determined from the final state analysis.


 Please wait while we load your content...
Please wait while we load your content...