Spatial quenching of a molecular charge-transfer process in a quantum fluid: the Csx–C60 reaction in superfluid helium nanodroplets†
Abstract
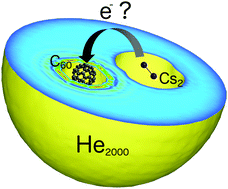
A recent experimental study [Renzler et al., J. Chem. Phys., 2016, 145, 181101] on superfluid helium nanodroplets reported different reactivities for Cs atoms and Cs2 dimers with C60 fullerenes inside helium droplets. Alkali metal atoms and clusters are heliophobic, therefore typically residing on the droplet surface, while fullerenes are fully immersed into the droplet. In this theoretical study, which combines standard methods of computational chemistry with orbital-free helium density functional theory, we show that the experimental findings can be interpreted in the light of a quenched electron-transfer reaction between the fullerene and the alkali dopant, which is additionally hindered by a reaction barrier stemming from the necessary extrusion of helium upon approach of the two reactants.

 Please wait while we load your content...
Please wait while we load your content...