Regarding the torsional flexibility of the dihydrolipoic acid's pharmacophore: 1,3-propanedithiol†
Abstract
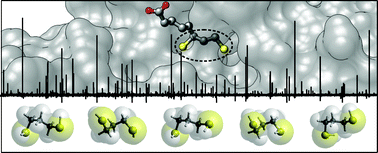
The conformational space of antioxidant dihydrolipoic acid has been explored through the investigation of its pharmacophore, 1,3-propanedithiol. Five of the possible 25 non-equivalent isomers (namely: gGGg′, gGGg, g′AGg, gAGg and g′AGg′) were observed in the 59.6–74.4 GHz frequency region using free-jet absorption rotational spectroscopy. Furthermore, for three of them, the 34S mono-substituted isotopologues were also detected in natural abundance. Theoretical simulations show that the balance of steric and electronic intramolecular interactions arises on a shallow conformational potential energy surface and suggest that in polar solvents the flexibility of the dithiol chain is greater than that in the isolated phase.


 Please wait while we load your content...
Please wait while we load your content...