Photosensitive microgels containing azobenzene surfactants of different charges†
Abstract
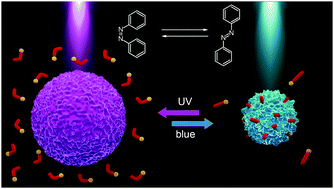
We report on light sensitive microgel particles that can change their volume reversibly in response to illumination with light of different wavelengths. To make the anionic microgels photosensitive we add surfactants with a positively charged polyamine head group and an azobenzene containing tail. Upon illumination, azobenzene undergoes a reversible photo-isomerization reaction from a trans- to a cis-state accompanied by a change in the hydrophobicity of the surfactant. Depending on the isomerization state, the surfactant molecules are either accommodated within the microgel (trans-state) resulting in its shrinkage or desorbed back into water (cis-isomer) letting the microgel swell. We have studied three surfactants differing in the number of amino groups, so that the number of charges of the surfactant head varies between 1 and 3. We have found experimentally and theoretically that the surfactant concentration needed for microgel compaction increases with decreasing number of charges of the head group. Utilization of polyamine azobenzene containing surfactants for the light triggered remote control of the microgel size opens up a possibility for applications of light responsive microgels as drug carriers in biology and medicine.

 Please wait while we load your content...
Please wait while we load your content...